Understanding Intestinal Barrier Function in Autoimmune Disease
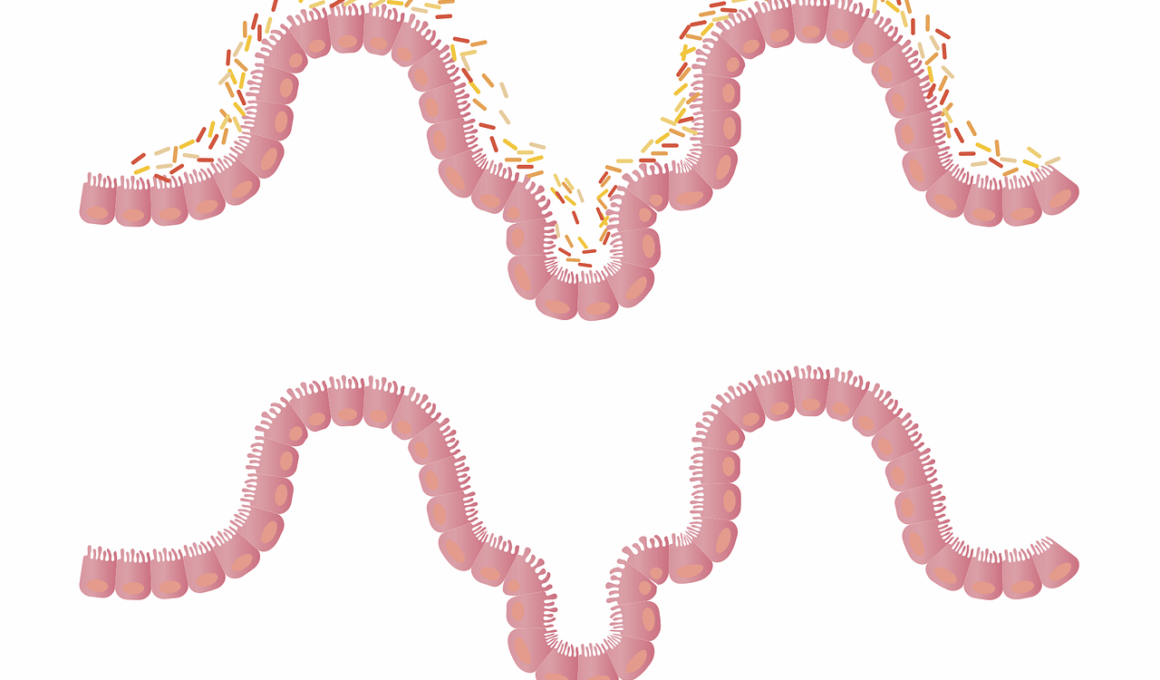
The gut microbiome plays a critical role in maintaining intestinal barrier function, which is essential in preventing autoimmune diseases. This barrier is a selective barrier that controls what enters the bloodstream from the digestive tract. When this barrier is compromised, substances like toxins and unprocessed food particles can leak into the bloodstream. This condition, often referred to as “leaky gut,” can trigger an immune response that may lead to the development of autoimmune conditions. The relationship between the gut microbiome and immune health is intricate and complex, influenced by factors such as diet, lifestyle, and individual health history. Research has indicated that maintaining a balanced microbiome can support the integrity of the intestinal barrier. A healthy gut microbiome consists of diverse microbial species that help digest food, synthesize vitamins, and protect against harmful pathogens. Factors such as antibiotics, unhealthy diets, and chronic stress can disrupt this balance, leading to dysbiosis. Overall, understanding the function of the intestinal barrier and its connection to autoimmune diseases is crucial for developing preventive strategies.
The Role of Gut Microbiome in Autoimmune Diseases
The gut microbiome’s influence on autoimmune diseases is a growing field of study. Autoimmune diseases occur when the immune system mistakenly attacks the body’s own tissues, causing inflammation and damage. Studies have shown that an imbalanced gut microbiome may contribute to the onset of these conditions by altering immune responses. For instance, certain bacterial species can produce metabolites that influence immune signaling pathways. This microbiome-derived signaling can lead to chronic inflammation, which is a hallmark of autoimmune diseases. Furthermore, the gut microbiome’s composition can be influenced by dietary choices, which underscores the importance of nutrition in gut health. Nutrient-rich diets that are high in fiber, prebiotics, and probiotics can foster a healthy microbiome, thereby supporting intestinal barrier integrity. Conversely, diets high in sugar and processed foods can promote dysbiosis, increasing the risk of autoimmune disorders. Interventions focusing on restoring gut health through dietary modifications are being explored as potential therapies for individuals with autoimmune diseases. By understanding these relationships, healthcare providers can help manage and potentially prevent autoimmune conditions effectively.
In addition to diet, lifestyle factors also play a significant role in shaping the gut microbiome and its impact on autoimmune diseases. Stress, for instance, has been shown to negatively affect gut health. Chronic stress can lead to hormonal changes that may compromise the intestinal barrier, leading to increased permeability. Physical activity is another crucial factor; regular exercise has been linked to improvements in gut microbiome diversity and richness. This diversity is vital for optimal immune system function, and a sedentary lifestyle may promote dysbiosis. Environmental factors, including exposure to pollutants and chemicals, can also disturb gut bacteria. Furthermore, the timing of food intake may impact the microbiome and overall immune health, suggesting that circadian rhythms could affect gut homeostasis. Sleep patterns and quality are additional contributors to gut health, as poor sleep has been associated with increased inflammatory markers. Recognizing these lifestyle factors is essential for developing holistic approaches to manage autoimmune diseases effectively. Comprehensive strategies combining dietary, physical, and psychological interventions could potentially enhance gut health and immune function.
Potential Therapies Targeting Gut Microbiome
Interventions aimed at modulating the gut microbiome are emerging as potential therapies for autoimmune diseases. Probiotics, which are live beneficial bacteria, are being studied for their ability to restore microbiome balance, enhance intestinal barrier function, and modulate immune responses. These probiotics can be consumed through fermented foods or supplements and may help prevent dysbiosis. Prebiotics, which are non-digestible fibers that feed beneficial gut bacteria, are equally important. Incorporating prebiotic-rich foods such as garlic, onions, and bananas can help promote the growth of beneficial bacteria, supporting a healthy microbiome. Fecal microbiota transplantation (FMT) has also gained attention as a radical therapeutic approach, showing promising results in certain conditions. FMT involves transferring stool containing healthy microbiota from a donor to a recipient to restore microbial balance. While still in research stages, this therapy could offer significant benefits to those with serious dysbiosis-related autoimmune diseases. However, further investigations are necessary to understand the safety, optimal protocols, and long-term effects of such interventions. Combining these therapies with established medical treatments may provide a more effective holistic approach to managing autoimmune diseases.
Research continues to reveal the complexity of gut microbiome interactions with the immune system. The gut-associated lymphoid tissue (GALT) is a crucial player in maintaining immune tolerance and is significantly influenced by gut microbiota. Disruption in this relationship can lead to an inappropriate immune response, contributing to autoimmune pathologies. Moreover, the gut microbiota regulates local and systemic inflammation, which ties into the pathogenesis of autoimmune diseases. For instance, certain gut bacteria may promote the production of regulatory T cells, which are essential for maintaining immune balance. The effect of diet on GALT and its microbiome interactions underlines the potential for dietary strategies in prevention and management. As more evidence emerges, the development of personalized nutrition plans based on individual microbiome profiling could enhance therapeutic interventions. The role of diet and gut health in autoimmune disease management exemplifies how interconnected our body systems are. Future research is essential to identify specific microbial species and their metabolites that might serve as biomarkers for diagnosing or predicting autoimmune conditions.
Conclusion: The Future of Gut Health and Autoimmune Diseases
Ultimately, understanding the intricate relationship between the gut microbiome and autoimmune diseases opens doors to new therapeutic possibilities. Research is ongoing to explore targeted treatments that resonate with the microbiome’s composition and function. For individuals at risk of autoimmune diseases, maintaining gut health through diet, lifestyle, and possibly microbiome-targeted therapies may serve as a preventive strategy. Regular monitoring of gut health is equally crucial as it can provide insights into broader immune system functioning. The exploration of microbial diversity, the effects of diet on microbial ecosystems, and innovative interventions will shape future healthcare approaches. Collaboration across disciplines, including immunology, nutrition, and microbiology, will foster holistic strategies aimed at managing and preventing autoimmune diseases. Furthermore, increased awareness of the gut’s role in systemic health will empower individuals to take proactive measures towards their wellness. Ultimately, as we expand our understanding of the gut microbiome, we embark on a journey to improve health outcomes, paving the way for potential breakthroughs in managing autoimmune diseases effectively.
In summary, the gut microbiome’s function in health and disease cannot be understated. It offers promising avenues for the treatment and management of autoimmune diseases. Maintaining a healthy intestinal barrier is essential in preventing leaky gut syndrome and subsequent immune dysregulation. Therefore, educating patients about their gut health could be vital in comprehensive autoimmune disease management. Testing and understanding one’s gut microbiome may become standard practices in personalized medicine. By utilizing cutting-edge research and clinical findings, we may better inform individuals and healthcare professionals about the critical importance of gut microbiome in autoimmune disease etiologies and treatment strategies. The convergence of dietary adherence, lifestyle management, and emerging therapies holds great potential for improving patient outcomes. Moving forward, continued research will illuminate more about the gut-brain-axis, inflammation, and immune responses. Each layer of insight gained will enhance our collective ability to combat autoimmune diseases. As we integrate this knowledge into clinical practice, we may not only improve treatment efficacy but also help individuals reclaim their health and well-being. The gut microbiome deserves recognition as a key player in the ongoing quest for effective healthcare solutions.
Finally, future research directions may include studying microbiome-immune interactions from infancy through aging. Understanding how environmental exposures, genetic predispositions, and lifestyle factors influence gut microbiota early in life could provide cues for preventing autoimmune diseases. Moreover, utilizing advanced technologies like metagenomics, which studies the genetic material of microbial communities, is essential for comprehensively understanding how these microbes affect host health. Therefore, refining our approaches to studying the gut microbiome will be essential to unveil potential intervention strategies. Integrating personalized medicine with gut health may unlock innovative treatment paradigms geared toward autoimmune disease mitigation. As the understanding of gut microbiome strategies matures, the landscape of autoimmune disease management may evolve significantly. Collaborations with dietitians, researchers, and healthcare providers will encourage a multidisciplinary approach towards patient care. These combined efforts can lead to significant advancements in therapeutic options and outcomes for individuals with autoimmune diseases. Ultimately, recognizing the gut’s profound impact on overall health will play a transformative role in healthcare and wellness strategies moving forward.