How Antibiotic Exposure Alters Gut Microbiome and Metabolism in Children
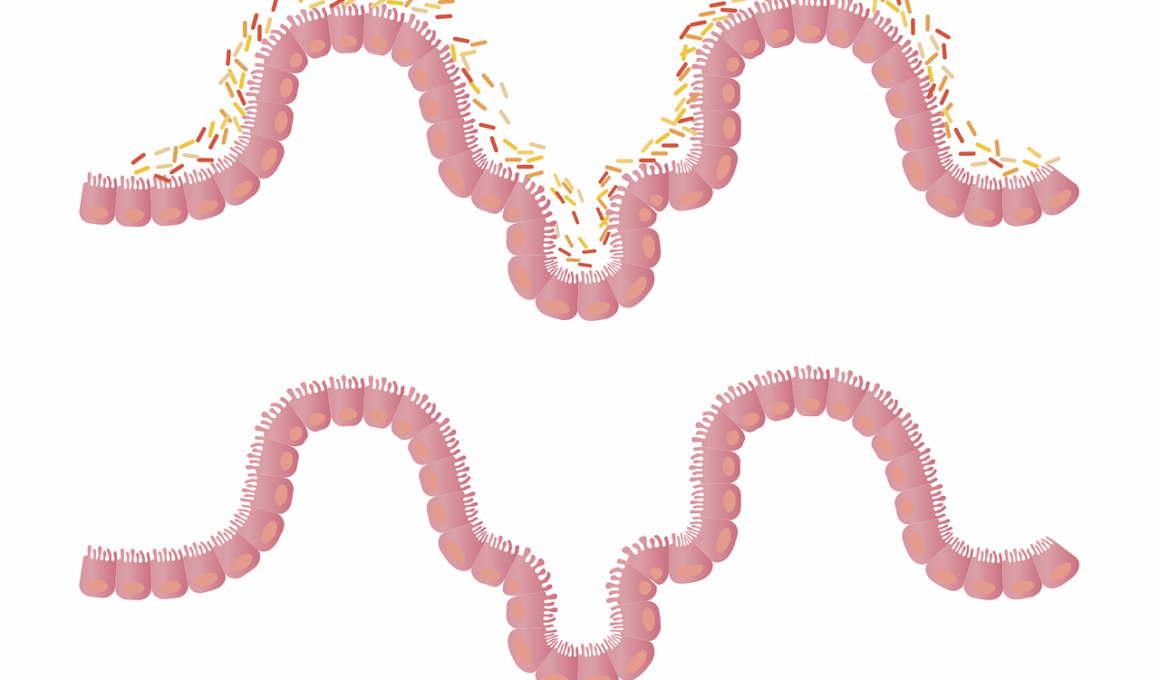
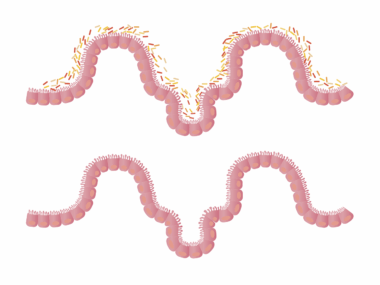
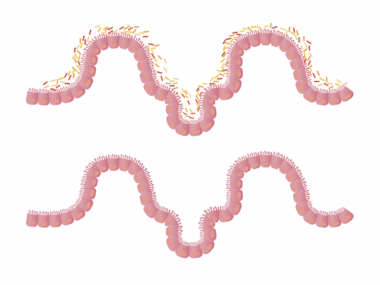
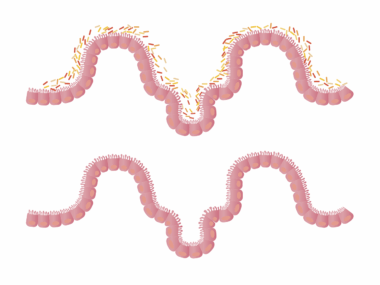
Antibiotic exposure during early childhood significantly affects gut microbiome composition, leading to various health issues. In children, the gut microbiome plays a crucial role in digestion, immune function, and metabolism. Antibiotics disrupt the delicate balance of beneficial bacteria in the gut, directly impacting metabolic processes. The human gut houses trillions of microorganisms, primarily bacteria that regulate metabolic pathways, produce vitamins, and modulate immune responses. When antibiotics are administered, they do not only target harmful bacteria but frequently eliminate beneficial ones, leading to dysbiosis. Dysbiosis is characterized by reduced diversity and altered composition of gut microbiota, which can result in increased susceptibility to infections, obesity, and metabolic disorders. Therefore, understanding the long-term impacts of antibiotic exposure on gut microbiome is vital. Longitudinal studies indicate that children exposed to antibiotics have an increased risk of obesity and related metabolic diseases later in life. This connection emphasizes the need for cautious antibiotic use, especially in young populations. Encouraging probiotic-rich diets post-antibiotic treatment might help mitigate some adverse effects on the microbiome and metabolic health.
The Role of the Gut Microbiome in Metabolism
The gut microbiome consists of a complex community of bacteria that plays a pivotal role in human metabolism. These microbes are responsible for breaking down complex carbohydrates and synthesizing essential nutrients, which contributes to overall health. Emerging research suggests that a healthy gut microbiome is associated with normal metabolic function, whereas an imbalanced microbiome is linked to obesity and metabolic syndrome. The interactions between the gut microbiota and host metabolism influence energy homeostasis, and when this balance is disrupted, it can lead to inflammatory responses affecting metabolism. Antibiotic-induced dysbiosis can decrease the microbiome’s ability to produce short-chain fatty acids, which are crucial for energy regulation and metabolism. Moreover, these changes can interfere with glucose metabolism and fat storage, potentially leading to excessive weight gain and related complications. Given these implications, maintaining a healthy gut microbiome is essential for optimizing metabolic health. Therefore, individuals, especially children, are encouraged to adopt diets rich in fiber and fermented foods to support their gut health. Understanding how our lifestyle affects microbiome composition can empower individuals to make informed decisions about their health.
A growing body of evidence highlights the link between antibiotic exposure, gut microbiome alterations, and metabolic health issues in children. When antibiotics are administered, they may have immediate and long-lasting effects, such as altering the microbial community structure. Furthermore, the timing of antibiotic exposure is critical; early-life disruption appears particularly disturbing as the gut microbiome is developing. Previous studies have shown that children given antibiotics have lower gut microbiome diversity compared to their non-exposed peers. This lack of diversity may predispose them to metabolic disorders in later life, such as obesity and type 2 diabetes. Research indicates that children receiving multiple rounds of antibiotics are at even higher risk of metabolic disturbances. These findings raise significant concerns about antibiotic stewardship in pediatric populations. Each course of antibiotics can lead to permanent changes in the microbiome, creating an opportunity for harmful bacteria to thrive. Strategies that include proper antibiotic use and the introduction of probiotics may help restore gut health. Addressing these concerns can lead to improved long-term health outcomes, affecting not just the individual but future generations as well.
Antibiotic resistance is a growing public health concern, exacerbated by the overuse and misuse of antibiotics. The impact of accounting for potential resistance in children’s health should not be overlooked. Overusing antibiotics can lead to resistant strains of bacteria that remain even after treatment. This resistance can make future infections more challenging to treat, compounding the risks associated with altered gut microbiomes. It is essential to comprehend how these resistant strains can also affect metabolic processes. They may outcompete beneficial bacteria, further destabilizing the gut ecosystem and leading to a cascade of health issues. This calls for a greater emphasis on judicious antibiotic use among healthcare providers and caregivers. Children should only receive antibiotics when absolutely necessary, and alternative treatments should be considered. Public health strategies should focus on promoting basic hygiene practices and vaccination to reduce infection rates and the need for antibiotics. The convergence of antibiotic use and metabolic health necessitates a multi-faceted approach to reduce unnecessary prescriptions, thus protecting the fragile gut microbiome throughout childhood.
Restoration of Gut Microbiome Post-Antibiotics
The restoration of the gut microbiome following antibiotic treatment is imperative for maintaining health. Unfortunately, it can take time for the microbiome to recover completely after antibiotic therapy. Some studies suggest that specific dietary interventions can expedite this recovery. Probiotics, which contain live beneficial bacteria, may help restore balance to the microbiome when consumed after antibiotic therapy. Meanwhile, a diet high in fiber can encourage the growth of beneficial strains and improve overall gut health. Fermented foods containing live cultures can serve as another beneficial addition to a post-antibiotic diet. Parents and caregivers can play a critical role in facilitating microbiome recovery by incorporating such foods into children’s diets. Additionally, understanding the importance of prebiotics—which serve as food for beneficial gut bacteria—can enhance microbiome resilience. Foods rich in prebiotics include garlic, onions, and bananas. By intentionally promoting a diverse microbiome, parents can help mitigate the adverse effects of previous antibiotic exposure. Awareness about the role of food in gut health can empower families to make health-centric decisions that will benefit children in the long term.
In conclusion, antibiotic exposure has a profound impact on the gut microbiome and, subsequently, metabolic health in children. The loss of microbial diversity can lead to metabolic disorders and obesity later in life. As awareness of these issues grows, the importance of prudent antibiotic use becomes increasingly vital. It is essential for healthcare providers to weigh the benefits against risks when prescribing antibiotics to children. Parents and caregivers must also be educated regarding the potential consequences of antibiotic therapies on their child’s gut health. Encouraging healthy dietary habits can help mitigate the adverse effects of antibiotics. Next, further research is required to fully understand the connection between microbiome health and metabolic diseases. Longitudinal studies will be essential to create best practices in pediatric care that incorporate microbiome considerations. Probiotic supplementation and focus on dietary diversity can pave the way for healthier outcomes in children exposed to antibiotics. Furthermore, promoting awareness of these subjects can encourage societal change regarding antibiotic practices. With concerted effort, we can protect and restore the critical balance of our microbiomes.
Strategies that include proper antibiotic use and the introduction of probiotics may help restore gut health. Addressing these concerns can lead to improved long-term health outcomes, affecting not just the individual but future generations as well.