The Promise of Gut Microbiome Modulation in Autoimmune Disease Treatment
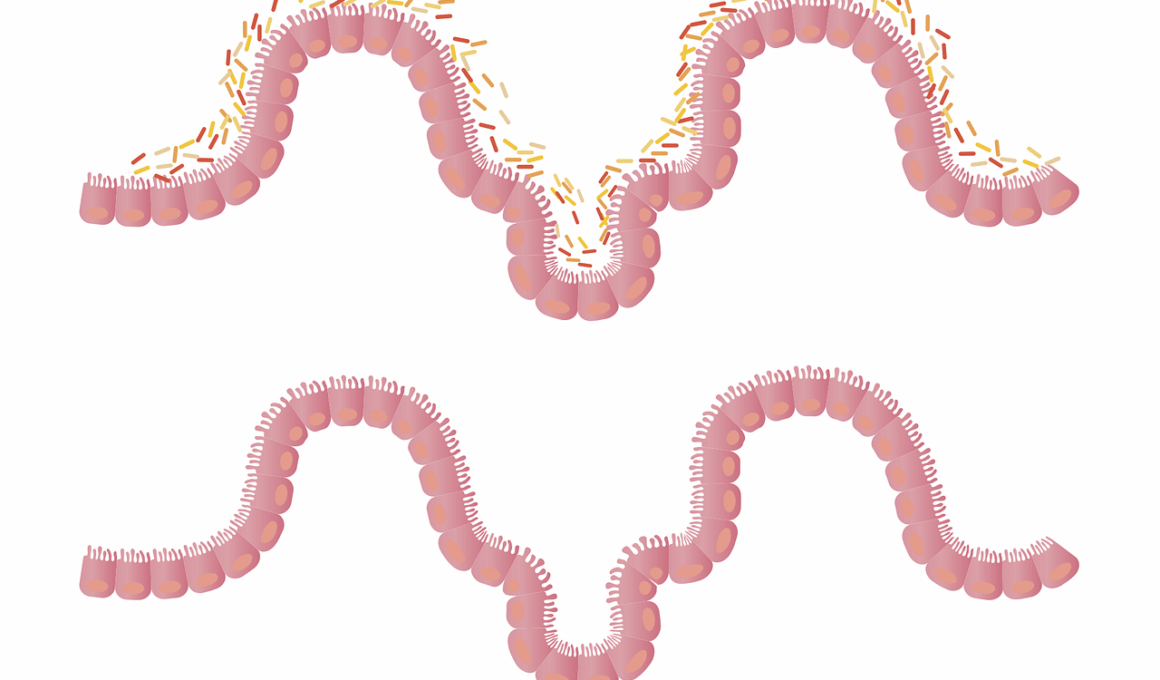
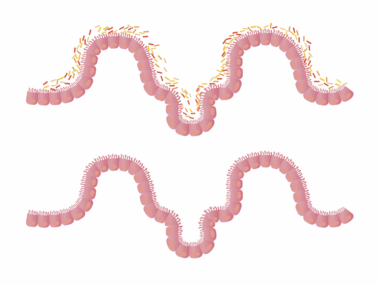
The gut microbiome plays a critical role in human health, influencing both metabolic and immune functions. Notably, a balanced microbiome contributes to a robust immune system, while dysbiosis—an imbalance of gut bacteria—may trigger autoimmune disorders. Research increasingly indicates that these autoimmune conditions connect to gut health. Autoimmune diseases like rheumatoid arthritis, lupus, and multiple sclerosis have been associated with specific aberrations in gut microbial composition. Therapeutic approaches targeting these imbalances may hold promise for improving patient outcomes. Certain probiotics, prebiotics, and dietary modifications can foster a healthier microbiotic environment. Early studies have demonstrated that restoring microbial diversity may enhance immune regulation, potentially alleviating symptoms in autoimmune disease patients. Understanding the connection between the gut and immune system could unlock new avenues for non-invasive, effective therapies. Continuing research aims to identify specific microbial strains beneficial for modulating autoimmune responses, a necessary step toward personalized treatment.Gut microbiome modulation may lead to innovative therapeutic strategies that transform how autoimmune diseases are managed.
Preliminary investigations reveal the gut microbiome’s extensive influence on the immune system, suggesting a complex interplay between gut bacteria and autoimmune diseases. Inflammation often precedes autoimmune conditions and appears to correlate with altered gut microbiota. A study found that specific strains of bacteria, such as Lactobacillus, can ameliorate inflammatory responses in autoimmune models. Investigating the species composition of gut microbiomes in patients with autoimmune diseases can help identify biomarkers that predict disease onset or escalation. Furthermore, understanding which microbiota promote or impede inflammatory pathways offers potential targets for intervention. Therapeutic modulation of the gut microbiome through targeted microbial supplements could serve as an adjunctive treatment alongside traditional therapies. For instance, a recent study indicated that certain probiotics could reduce disease activity in rheumatoid arthritis patients, showcasing the microbiome’s therapeutic potential. By harnessing this knowledge, clinicians can develop tailored probiotic formulations aimed at reestablishing healthy gut flora. Additionally, this approach presents a lifestyle modification strategy supporting overall immune health and enhancing patients’ quality of life.
The Role of Diet in Gut Health
The role of diet in supporting a healthy gut microbiome cannot be overstated, particularly for those dealing with autoimmune diseases. Certain foods nourish beneficial gut bacteria, promoting microbial diversity and reducing inflammation. Diets rich in fiber, such as fruits, vegetables, and whole grains, provide essential nutrients for gut microbiota. Additionally, fermented foods like yogurt and kefir introduce live cultures that can help restore balance. Eliminating processed foods and excessive sugars may also support a healthier gut environment. The Mediterranean diet, characterized by healthy fats and an abundance of plant-based foods, is often recommended for its anti-inflammatory properties. Studies suggest that adhering to this dietary pattern may significantly improve symptoms associated with autoimmune conditions. Moreover, consistent meal patterns and avoiding known allergens or intolerances can further mitigate gut-related issues. Incorporating omega-3 fatty acids, found in fish and flaxseeds, has shown promise in reducing inflammation. Ultimately, dietary modifications serve as both a preventive measure and a complementary treatment for those with autoimmune diseases, emphasizing the importance of nurturing gut health.
In addition to dietary approaches, research continuously explores the potential of prebiotics in managing autoimmune diseases. Prebiotics are non-digestible food components that promote the growth of beneficial gut bacteria. This process enhances microbial diversity, which is crucial for maintaining immune homeostasis. Ingredients like inulin and fructooligosaccharides, prevalent in garlic, onions, and asparagus, have been associated with positive health outcomes. Clinical trials examining the efficacy of prebiotics in managing conditions like Crohn’s disease or ulcerative colitis demonstrate promising results. By enhancing gut health, prebiotics may mitigate symptoms and improve the efficacy of conventional treatments. Furthermore, emphasizing dietary sources of prebiotics aids in achieving a holistic approach to gut health. With more studies underway, the relationship between prebiotics and inflammation holds exciting potential for future interventions. Understanding how these inputs reshape the gut microbiome could lead to new dietary recommendations tailored for autoimmune patients. Thus, ongoing research into prebiotics could significantly impact management strategies for autoimmunity, emphasizing the intricacies of our gut and immune interactions.
Probiotic Intervention Studies
Probiotic interventions represent a novel approach to treating autoimmune diseases by reshaping the gut microbiome. Convenience, safety, and efficacy make probiotics an attractive option for disease management. Recent clinical trials have examined the influence of specific probiotic strains, such as Lactobacillus rhamnosus and Bifidobacterium bifidum, on markers of inflammation in autoimmune patients. These studies often reveal that patients receiving tailored probiotic therapies experience fewer symptoms and improved quality of life. By investigating the underlying mechanisms, researchers aim to understand how probiotics modulate immune functions and equilibrium within the gut environment. Oral probiotic supplementation can support the integrity of the gut lining, potentially reducing the risk of systemic inflammation. The findings emphasize a possible role for probiotics as adjunct treatments to conventional therapies, with the potential to decrease reliance on immune-suppressing medications. However, variations in individual responses pose challenges regarding optimal strains, dosages, and treatment durations. Continued research in this area will provide critical insights into feasible probiotic applications for different autoimmune conditions while enhancing overarching treatment strategies.
Research also highlights the necessity of individualized approaches to gut microbiome modulation in autoimmune diseases. Individual responses to dietary interventions, probiotics, and prebiotics can differ significantly based on genomic and lifestyle factors. Thus, tailoring strategies to each patient’s unique microbiome composition may optimize treatment outcomes. Microbiome analysis through stool samples allows researchers to identify specific bacterial populations and their potential therapeutic implications. Furthermore, leveraging advances in genomic sequencing and metabolomics enables a comprehensive understanding of how individual microbiomes function. These insights expand the possibilities for personalized medicine, particularly in the realm of autoimmune care. Tailored interventions—whether through dietary modifications or targeted microbe administration—could potentially lead to more effective and sustainable outcomes. It is essential to incorporate patient history and preferences in developing these personalized strategies, ensuring holistic care. Ultimately, understanding the nuances of the gut microbiome presents an exciting frontier for improving treatment approaches in autoimmune diseases and changing the paradigm of patient care.
Future Directions in Research
The future of gut microbiome modulation in the context of autoimmune disease treatment is promising yet complex. Multidisciplinary research efforts are key to unlocking the full potential of microbiome therapeutics. Studies combining microbiology, immunology, nutrition, and personalized medicine offer a comprehensive view of interventions in autoimmune diseases. Additionally, large-scale, longitudinal studies are necessary to establish definitive relationships between microbiome characteristics and disease progression. Identifying specific microbial markers can guide interventions, paving the way for precision medicine in autoimmune care. Future research should also explore the synergistic effects of different therapeutic agents, including probiotics, prebiotics, and dietary strategies. Interventions aimed at altering gut permeability and fostering beneficial microbial environments will likely take precedence as the complexities of gut-brain interactions are further understood. Investigating the impact of gut microbiome health on mental well-being remains an intriguing area of exploration. As research progresses, evolving insights will not only improve therapeutic strategies for autoimmune diseases but also enhance our understanding of health and disease from a holistic perspective.
In conclusion, addressing gut microbiome alterations presents an exciting avenue for managing autoimmune diseases. With substantial evidence supporting the microbiome’s role in immune function, exploring dietary and probiotic interventions could lead to transformative treatment options. By focusing on modulating the gut environment, healthcare providers may offer patients better outcomes, reduced symptoms, and improved quality of life. Future research endeavors promise continued advancements in the field of microbiome science, tailored therapeutic approaches, and insights into preventive care. These strategies emphasize the importance of a balanced microbiome in sustaining overall health. Integrating gut health into comprehensive treatment plans highlights the need for individualized patient-centered care. As research uncovers more intricate relationships between the microbiome and autoimmunity, the potential for innovative therapies becomes increasingly evident. Building upon current findings, it is essential to foster collaborative approaches among clinicians, researchers, and patients in advancing gut microbiome science. Overall, the implications of gut microbiome modulation for autoimmune disease treatment, if correctly harnessed, could revolutionize patient management and support the quest for healthier lives.