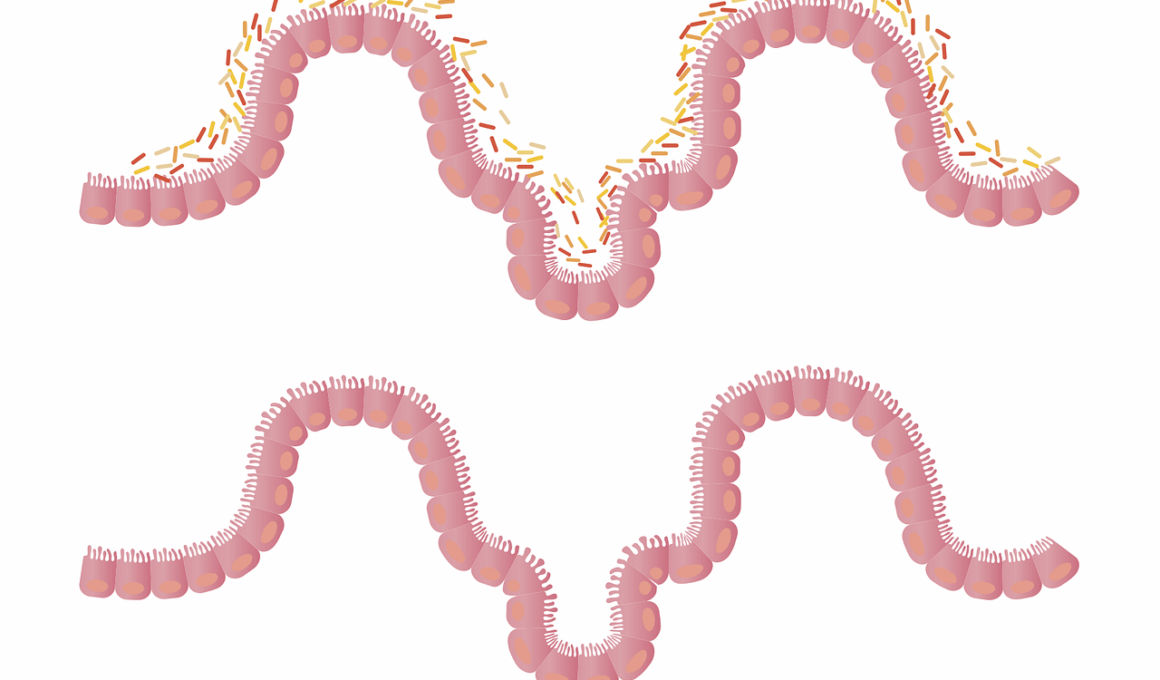
The Role of Antibiotics in Disrupting Gut Microbiota
Antibiotics, while crucial for treating bacterial infections, significantly disturb the delicate balance of gut microbiota. The gut microbiome comprises trillions of microorganisms, including bacteria, fungi, and viruses, essential for digesting food, synthesizing vitamins, and maintaining a robust immune system. When antibiotics are administered, they can indiscriminately kill both harmful and beneficial bacteria. This disruption leads to a variety of gastrointestinal issues, such as diarrhea and infections due to opportunistic pathogens. Research indicates that even short courses of antibiotics can lead to long-lasting changes in the gut microbiome composition. Over time, these alterations can increase vulnerability to chronic diseases, including obesity, diabetes, and inflammatory bowel disease. Restoring gut flora after antibiotic treatment is challenging, as the recovery process varies among individuals. Probiotics and prebiotics have emerged as potential strategies to help restore the microbial balance. However, it’s crucial to approach the use of these supplements cautiously. Understanding the specific impact of different antibiotics on gut health will aid in developing more sustainable treatment protocols that minimize risks associated with microbiota disruption. Individuals should remain aware of their antibiotic use and the subsequent effects on their gut health.
Recent studies emphasize that antibiotics can foster an environment where pathogenic bacteria thrive. Disruptions in gut microbiota can result in dysbiosis, characterized by an imbalance of microbial populations. Such imbalances can lead to symptoms ranging from mild gastrointestinal disturbances to severe health complications. One notable example is Clostridium difficile infection, a serious condition often triggered by antibiotic therapy. In patients with altered gut flora, C. difficile can proliferate, leading to debilitating diarrhea and other complications. Moreover, the long-term effects of antibiotics on gut health are still under investigation. Some studies suggest that repeated or prolonged antibiotic use can lead to irreversible changes in gut flora. This phenomenon raises concerns regarding the over-prescription of antibiotics and their impact on public health. Patients should have open conversations with healthcare professionals about the necessity of antibiotics. Additionally, healthcare systems must prioritize responsible antibiotic prescribing practices to preserve microbiome diversity. Preventative measures, including vaccination and optimal hygiene, can reduce the reliance on antibiotics. Overall, understanding the complex interactions between antibiotics and gut microbiota is vital for implementing effective strategies for better health outcomes.
Impact on Immune Function
Antibiotics not only alter gut microbiota but also influence immune function significantly. A healthy gut microbiome plays a critical role in training the immune system to distinguish between harmful and benign entities. When antibiotics disturb this ecosystem, the immune system’s ability to function optimally may be compromised. The gut-associated lymphoid tissue (GALT), an essential component of the immune system, relies heavily on microbial interactions to develop appropriately. Studies show that antibiotic-induced changes in gut flora can result in increased inflammation and a higher susceptibility to autoimmune disorders. Furthermore, the diversity within the gut microbiome is crucial for maintaining immune tolerance, a state that prevents unnecessary immune responses against non-threatening antigens. Inadequate microbial diversity, often resulting from antibiotic use, can lead to dysregulated immune reactions. The implications of these changes highlight the importance of fostering a balanced gut microbiome. Integrating gut health into overall health strategies can ensure better immune responses. Patients are encouraged to maintain a healthy lifestyle, including a balanced diet rich in fiber and fermented foods, to support microbiome resilience and enhance immune system functionality after antibiotic treatment.
Another essential aspect is the timing and type of antibiotic administered. Broad-spectrum antibiotics can significantly impact the gut microbiome compared to narrow-spectrum antibiotics, which target specific pathogens while sparing beneficial bacteria. The indiscriminate action of broad-spectrum antibiotics can lead to an inability to regain the original microbial community post-treatment. Research is ongoing to understand these differential impacts better. Preventive measures, such as the use of narrow-spectrum antibiotics when appropriate, can help mitigate disruption to gut flora. Developing antibiotics that selectively target harmful bacteria without impacting beneficial ones could be revolutionary in maintaining gut health during treatment. The ongoing exploration of alternative treatments, including bacteriophage therapy and immunotherapy, offers exciting prospects for the future of medicine. These methods may provide ways to combat infections while preserving gut microbiota integrity. Effective communication among healthcare providers, researchers, and patients is essential for fostering a deeper understanding of the intricate relationships between antibiotics and gut health. Ultimately, more controlled usage of antibiotics will enhance both individual health outcomes and the broader struggle against antibiotic resistance in the healthcare system.
Restoring Gut Health After Antibiotics
Following antibiotic treatment, many individuals seek ways to restore their gut health. Probiotics, containing live beneficial bacteria, have gained popularity as a potential solution. They aim to replenish the gut microbiota that antibiotics may have diminished. However, research suggests that not all probiotics are equally effective; strain specificity matters. Certain strains may be better suited to rebalance the gut microbiome after antibiotic use. Additionally, prebiotics, which are non-digestible fibers that feed beneficial microorganisms, can further support gut health. Foods rich in prebiotics include bananas, onions, and garlic. Consuming a diverse range of fruits and vegetables daily can enhance gut microbiota diversity and aid in recovery. It’s crucial to consult a healthcare professional before starting any new supplement regimen. Moreover, lifestyle factors play an essential role in gut recovery, including adequate hydration, regular exercise, and stress management. Mindfulness practices and yoga can contribute to overall well-being and potentially influence gut health positively. Each individual’s response to antibiotics varies, so personalized approaches may yield the best results. Ultimately, promoting awareness of gut health post-antibiotic treatment is vital for long-term wellness.
In addition to dietary and lifestyle changes, ongoing research into gut health sheds light on innovative therapies. Fecal microbiota transplantation (FMT) is one such intervention gaining traction in clinical settings. This procedure involves transferring fecal material from a healthy donor into a patient’s gut, aiming to restore microbial diversity and combat dysbiosis. FMT has shown promise in treating recurrent C. difficile infections and other gastrointestinal disorders. Nonetheless, this therapy raises ethical and safety considerations, warranting thorough study and regulatory oversight. Other therapies, like synbiotics, which combine probiotics and prebiotics, are being investigated for their potential to enhance gut health following antibiotic use. The focus on personalized medicine enables tailored interventions based on individual microbiome compositions. By fostering a deeper understanding of the microbiome’s role in health, researchers aim to create targeted approaches to mitigate the adverse effects of antibiotics. Maintaining dialogue among scientists, clinicians, and patients will be essential to accelerate these developments. The systematic study of gut microbiota is pivotal for not only combating infections but also enhancing overall health and preventing future antibiotic-related complications.
Conclusion
In conclusion, the role of antibiotics in disrupting gut microbiota highlights the essential need for cautious usage. Understanding their impact on gut health is vital for informed medical decisions. Antibiotics, though life-saving, carry potential long-term consequences for gut flora and general well-being. A balanced gut microbiome is not only crucial for digestive health but also plays an integral role in immune function and disease prevention. As more individuals face the repercussions of antibiotic overuse, there is an urgent need to adopt responsible practices in their application. Healthcare professionals must assess the necessity of antibiotics carefully and consider the implications for gut microbiome health. Patients are encouraged to be proactive in discussing alternatives and preferences with their healthcare providers. By incorporating preventive measures and supportive interventions, such as probiotics and prebiotics, recovery from antibiotic disruption is feasible. Continued research and innovation will play a crucial role in developing therapies that target infections while preserving gut health. Fostering awareness about the interplay between antibiotics and gut microbiota will ultimately contribute to a healthier population. The journey towards understanding and repairing gut health continues, paving the way for better treatment strategies.
The growing body of research underscores the importance of addressing gut health not only immediately after antibiotic use but also as part of an integrated approach to overall health management. Patients are encouraged to maintain a holistic view of their health, considering factors such as diet, exercise, and stress management as interconnected elements influencing gut health. Collaboration with nutritionists and health coaches can provide personalized guidance to optimize gut-friendly choices. Furthermore, education about the potential negative effects of antibiotics on gut microbiota is crucial for both patients and healthcare providers. As awareness increases, more individuals will make informed decisions regarding antibiotic use, preventing unnecessary prescriptions and fostering healthy gut ecosystems. Public health initiatives focusing on the importance of microbiome health can significantly contribute to reducing the reliance on antibiotics. Innovations in microbiome research will likely yield novel insights into gut health, reinforcing the need for ongoing education and awareness among healthcare professionals and the general public alike. Ultimately, empowering individuals with knowledge about gut health can enhance their health outcomes and build a healthier society as a whole.