The Interplay Between Gut Fungi and Autoimmune Disorders
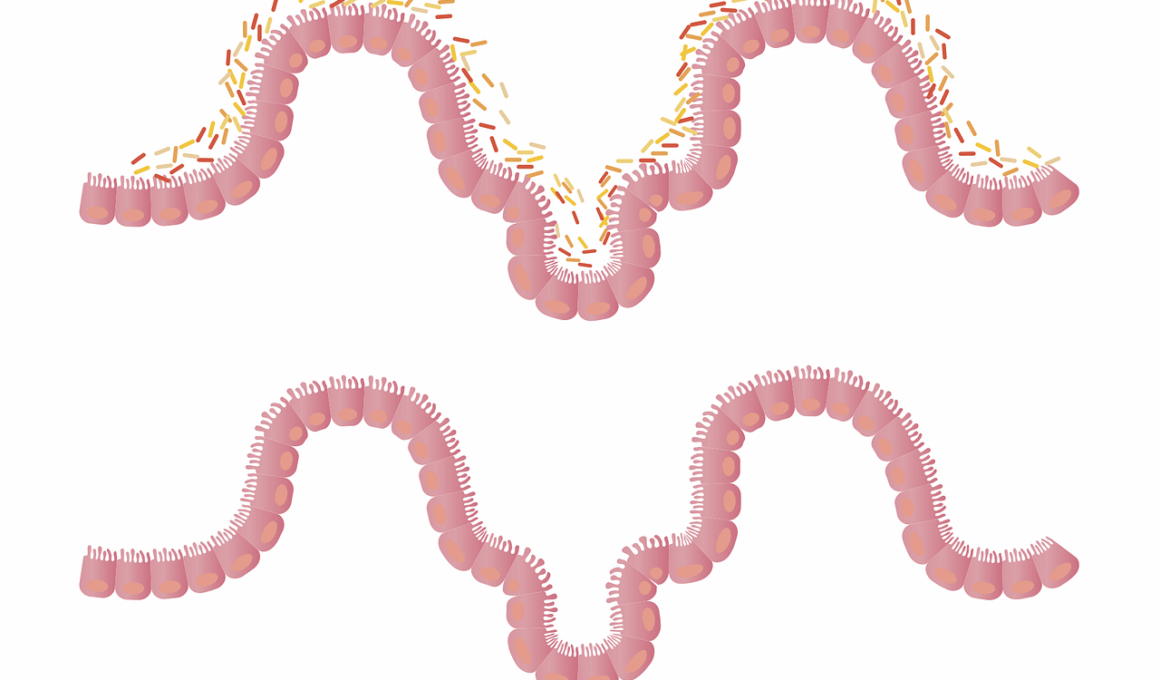
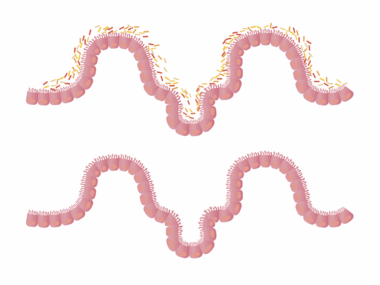
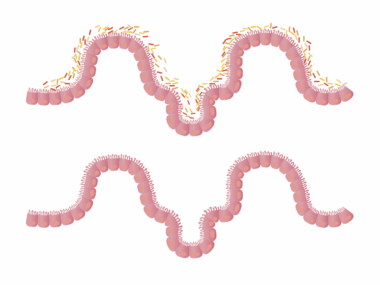
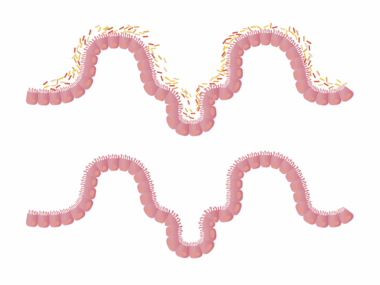
Understanding the gut microbiome is essential due to its significant role in health and disease. The gut microbiome comprises trillions of microorganisms, including bacteria, viruses, archaea, and fungi. These microbes can affect various bodily functions, influencing the immune response and metabolic processes. When the microbiome is disrupted, it can lead to dysbiosis, which is often linked to autoimmune diseases. Autoimmune disorders occur when the immune system mistakenly attacks healthy tissues, leading to inflammation and damage. Fungal species within the gut are increasingly being recognized for their roles in these conditions. Studies have shown that an overabundance of specific fungi can exacerbate autoimmune responses, creating an imbalance. The immune system’s interaction with these fungi is complex and can vary from person to person. Understanding these interactions provides insights into potential treatment options and preventive measures against autoimmune diseases. By studying the mechanisms of gut fungi in autoimmune conditions, researchers hope to identify new therapeutic targets that may help restore balance and improve patient outcomes. This exploration of the gut-brain axis will be crucial in developing personalized medicine for those affected by autoimmune disorders.
Research has begun to uncover the connection between gut health, especially the role of gut fungi, and autoimmune diseases. Recent studies illustrate how fungi, such as Candida, can influence inflammatory processes and immune responses. These microorganisms can produce metabolites that impact gut health and systemic inflammation. Autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and lupus, have shown associations with specific gut microbiome profiles. For instance, a lack of certain beneficial fungal species has been observed in patients with rheumatoid arthritis, indicating a potential protective role. Dysbiosis, characterized by an imbalance of gut fungi and bacteria, can lead to increased permeability of the gut lining, often referred to as leaky gut. This condition allows microbial products to enter the bloodstream, triggering immune responses that may contribute to autoimmune pathology. Understanding how to restore a healthy fungal balance in the gut could become a promising avenue for therapeutic intervention. This entails both dietary modifications and possibly even probiotic therapies that incorporate beneficial fungi strains. Further research is essential to define the mechanisms at play and develop effective treatment protocols for individuals grappling with these chronic diseases.
The Role of Diet in Gut Fungi and Autoimmunity
Diet plays a pivotal role in shaping the gut microbiome, affecting both fungi and bacteria populations. A diverse, fiber-rich diet can promote the growth of beneficial microbial species, which may combat inflammation and support immune function. Specific dietary components, like prebiotics and probiotics, can also influence gut health positively. Prebiotics, which are found in foods like bananas, garlic, and onions, serve as food for beneficial gut microbes. Conversely, overconsumption of sugar and processed foods can lead to dysbiosis, thereby promoting harmful fungus and bacteria. It’s been shown that Mediterranean and plant-based diets may reduce the risk of autoimmune diseases. Incorporating a wide variety of fruits, vegetables, nuts, and whole grains can help maintain a balanced gut mycobiome. Furthermore, fermented foods such as yogurt, sauerkraut, and kimchi are excellent sources of probiotics, aiding in gut health restoration. As we continue to understand the interplay between diet, gut fungi, and autoimmune diseases, dietary interventions could become a key component of treatment plans. Future research may illuminate specific foods that directly influence fungal populations, thus providing tailored dietary recommendations for autoimmune patients.
The gut-brain connection is another crucial aspect of understanding autoimmune diseases linked to dysbiosis. This connection illustrates how the gut microbiome communicates with the brain, influencing both emotional and physical health. Gut fungi contribute to this interplay, releasing metabolites that can affect neurotransmitter production and mood regulation. Studies suggest that a disruption in the gut microbiome can lead to both gut and brain inflammation, perpetuating the cycle of autoimmune responses. Patients with autoimmune disorders often report symptoms of anxiety and depression, highlighting the importance of a holistic approach to treatment. Addressing the balance of gut fungi could potentially mitigate these psychological symptoms. Researchers are investigating how restoring a diverse mycobiome may positively impact mental health alongside physical health. Cognitive functions, mood stabilization, and stress response could all benefit from improved gut health. This holistic view may encourage the integration of psychological therapies alongside dietary and lifestyle changes in managing autoimmune conditions. Understanding the gut-brain connection could lead to more comprehensive treatment interventions that address both mental and physiological symptoms of autoimmune disorders, ultimately improving patient quality of life.
Future Directions in Research
As research into the gut microbiome evolves, a deeper examination of the roles that fungi play in autoimmune diseases is becoming increasingly prominent. Future studies should focus on identifying specific fungal species and their metabolites that contribute to immune system modulation. Moreover, understanding how fungal infections complicate autoimmune conditions is crucial. Some autoimmune diseases may present worsened symptoms during fungal overgrowth. Identifying these relationships could open up new avenues for targeted therapies that aim to restore mycobiome balance. Techniques such as microbiome sequencing can provide insights into the diverse populations of fungi present in individuals with autoimmune disorders. Longitudinal studies will further reveal how these populations change over time and in response to treatments. Collaborative efforts among microbiologists, immunologists, and dietitians will be essential to develop multifaceted strategies for managing autoimmune diseases. This could include personalized dietary plans and targeted microbiome therapies. The exploration of fungi in the context of autoimmunity represents a fascinating frontier in medical research, one that could significantly enhance treatment effectiveness and patient outcomes. An interdisciplinary approach will facilitate much-needed advancements, paving the way for innovative therapies and management strategies.
The implications of restoring gut fungi balance extend to preventative strategies as well. By understanding the links between gut health and autoimmune conditions, it becomes possible to develop lifestyle habits that promote a healthy microbiome from a young age. Education about diet, probiotic intake, and the importance of maintaining a balanced microbiome is crucial in community health initiatives. Initiatives aimed at educating the public on the benefits of a diverse diet rich in fruits, vegetables, and whole grains may encourage healthier eating habits. Moreover, recognizing the symptoms of dysbiosis early can lead to proactive measures before serious autoimmune diseases develop. Encouraging regular check-ups and gut health screenings can facilitate early interventions. Additionally, incorporating physical activity and reducing stress levels enhances overall gut health. Integrating these principles into daily routines may diminish the likelihood of autoimmune diseases later in life. With ongoing research, the hope is to establish guidelines for maintaining gut health that provide comprehensive recommendations for prevention. Ultimately, fostering a culture of gut health awareness may empower individuals to take charge of their well-being, potentially leading to lower rates of autoimmune diseases in the future.
Conclusion
The connection between gut fungi and autoimmune disorders underscores the complexity of the gut microbiome and its influence on overall health. Ongoing research is critical in elucidating these relationships and their impact on immune regulation. As our understanding grows, we may find innovative therapies that focus on restoring mycobiome balance as a means to treat and prevent autoimmune diseases. The promise lies in integrating dietary, lifestyle, and microbiome therapies into a holistic approach to management. A proactive stance on gut health, informed by scientific discoveries, can empower individuals in their healthcare journeys. Future investigations will undoubtedly explore new pathways for therapeutics, leading to tailored solutions that address individual needs. By recognizing the importance of gut fungi, we pave the way for a more comprehensive understanding of autoimmune diseases. This knowledge may transform treatment protocols, emphasizing the role of diet and lifestyle in supporting a healthy microbiome. Encouraging ongoing conversations around gut health can inspire a cultural shift towards prevention, emphasizing proactive measures over reactive ones. With dedication to comprehending this interplay, there is significant potential for improving the quality of life for those affected by autoimmune conditions.
Through collaboration and innovation, the future of gut health research promises to yield important discoveries that enhance our understanding of diseases. By focusing on the interplay between gut fungi and autoimmunity, we can unlock new avenues for treatment and prevention strategies. Taking a comprehensive approach to gut health may encourage individuals to make informed lifestyle choices that promote their well-being. From diet to stress management, every aspect can contribute to a robust microbiome. As we forge ahead, the challenge lies in bridging the gap between research and practical applications in healthcare. Partnerships among various disciplines will be essential, as advancements in gut health will benefit from shared knowledge and expertise. This pursuit may lead to the development of personalized interventions designed to meet the unique needs of patients. Restoring balance within the gut mycobiome has the potential to revolutionize how we address autoimmune diseases. Continued exploration and investment in gut health research can illuminate the role of fungi, shifting paradigms in treatment. With sustained efforts, we can aspire to a future where autoimmune diseases are managed more effectively, fostering healthier lives and improved health outcomes for all individuals.